Chao-Jen Huang Quizzes 440
Sex and Age Differences Attune Clan of Vitamin D with Serum Triglyceride Levels
ane
Division of Endocrinology and Metabolism, Section of Internal Medicine, Wan Fang Hospital, Taipei Medical University, Taipei 11696, Taiwan
two
Division of Endocrinology and Metabolism, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei 11031, Taiwan
3
Section of Full general Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei 11031, Taiwan
iv
Graduate Institute of Clinical Medicine, Higher of Medicine, Taipei Medical University, Taipei 11031, Taiwan
5
Department of Medical Didactics and Research, Wan Fang Infirmary, Taipei Medical University, Taipei 11696, Taiwan
6
Department of Family Medicine, Wan Fang Infirmary, Taipei Medical Academy, Taipei 11696, Taiwan
vii
Cardiovascular Research Eye, Wan Fang Hospital, Taipei Medical Academy, Taipei 11696, Taiwan
8
Taipei Heart Establish, Taipei Medical University, Taipei 11031, Taiwan
*
Authors to whom correspondence should be addressed.
†
These authors accept equally contributed to this work and should be regarded as joint first authors.
Academic Editor: Jin-Rong Zhou
Received: 19 February 2022 / Revised: 4 March 2022 / Accepted: 9 March 2022 / Published: eleven March 2022
Abstruse
The sexual activity and age differences in the relationship between vitamin D and lipid levels remain unclear. This retrospective report investigated the correlations betwixt serum 25-hydroxyvitamin D levels and various biomarkers, along with the sex and age differences in these associations, among 573 men and 436 women during physical check-ups. The mean age of the report population was 51.4 years, and 66% of people had serum 25(OH)D levels below xxx ng/mL. People aged over 65 years had higher 25(OH)D levels than those younger than 65 years, and women had lower 25(OH)D levels than men. Younger historic period (odds ratio (OR) per year = 1.044, 95% CI, i.029–1.059, p < 0.0001), female sex (OR = 1.779, 95% CI, 1.149–2.755, p = 0.0097), and elevated serum triglyceride (TG) levels (OR per ane mg/dL = 1.005, 95% CI, one.002–one.007, p = 0.0002) were all contained take chances factors for vitamin D deficiency. Serum 25(OH)D levels were inversely associated with TG levels. The positive association between vitamin D deficiency and hypertriglyceridemia was pregnant in men (non in women) and in those aged between 50 and 65 years. In determination, younger individuals, women, and eye-anile men with hypertriglyceridemia are at higher risk of vitamin D deficiency.
1. Introduction
Vitamin D deficiency is a common disorder. The prevalence of vitamin D deficiency, defined as serum 25-hydroxyvitamin D (25(OH)D) levels <xxx ng/mL, is estimated to be thirty–fourscore% amongst adults worldwide [1,two]. Vitamin D not only regulates bone and mineral metabolism, simply also exerts various biological effects, including antioxidative, anti-inflammatory, antimicrobial, lipid-lowering, and cardiovascular protective effects [iii,iv,5]. In addition to osteoporosis, vitamin D deficiency has been reported to be associated with hypertension, diabetes mellitus, dyslipidemia, obesity, and metabolic syndrome [6,7,8]. Women and older adults are particularly vulnerable to vitamin D deficiency [9]. East Asian women usually avoid sunlight exposure to obtain off-white skin, putting them at a higher risk of vitamin D deficiency than men [10,11,12]. Malabsorption of cholecalciferol and ergocalciferol, a subtract in the cutaneous synthesis of vitamin D, reduced exposure to sunlight and an increase in the prevalence of liver and kidney disease all crusade serum 25(OH)D levels to decline with age [thirteen,14].
Humans obtain vitamin D through sunlight exposure and food intake. Vitamin D from cutaneous synthesis, or from the diet, is biologically inert. It is converted to 25(OH)D past 25-hydroxylase in the liver. Moreover, 25(OH)D, which is measured to determine an individual's vitamin D status, requires further hydroxylation in the kidneys to become bioactive vitamin D metabolite [4]. More often than not, ultraviolet B irradiation of 7-dehydrocholesterol in the skin contributes to the major source of vitamin D in humans since but a few foods, such every bit oily fish, egg yolks, and mushrooms, contain significant amounts of vitamin D [xv,16]. Therefore, variations in daylight throughout the year and latitude of residence both affect solar radiation, thus influencing vitamin D synthesis. A report involving 95,137 subjects in Korea revealed that the pattern of seasonal fluctuations of serum 25(OH)D levels and solar insolation was similar, suggesting that solar radiation is closely related to serum 25(OH)D levels [17]. The serum 25(OH)D levels are highest during late summer and lowest during late winter in northern countries [18,19,20]. Previous investigations take shown that the serum 25(OH)D levels in winter may be decreased by approximately 25–50% on average as compared to those in summertime [21,22]. Accordingly, seasonal variations should be taken into consideration when measuring serum 25(OH)D levels and treating vitamin D deficiency.
Although vitamin D deficiency is common and linked to many chronic disorders, show to recommend the routine screening of individuals for vitamin D deficiency is currently insufficient. Therefore, biomarkers to identify individuals at a high adventure for vitamin D deficiency could be clinically useful. Characteristic values of such biomarkers could increase a clinician's suspicion of the presence of vitamin D deficiency too every bit its clinical consequences or related comorbidities. Biochemistry is simple and widely used for assessing a patient's general condition, and the conventional biochemical parameters may include a biomarker of vitamin D deficiency. This study investigated the associations between serum vitamin D levels and diverse biomarkers, as well as the sex and age differences in these associations.
2. Methods
two.ane. Participants
From March 2018 to Feb 2020, we collected blood samples from individuals aged > 18 years who had received blood tests for vitamin D during their physical check-ups. Individuals with incomplete laboratory data were excluded. A total of 1009 participants were included in this report. The mean historic period was 51.4 years, and 56.7% of the participants were male person. They were divided into vitamin D sufficient and deficient groups at a cutoff 25(OH)D value of thirty ng/mL. Co-ordinate to the meteorological information of Taiwan, the solar irradiation was relatively lower from Nov to Apr (defined as extended winter) and higher from May to October (defined as extended summer) in Taipei [23]. This written report was approved past the Taipei Medical University Joint Institutional Review Lath (protocol code: N202006010).
ii.2. Biochemical Measurements
The following data were recorded: age, sexual practice, body weight, trunk mass index (BMI), complete blood count (CBC), serum levels of fasting plasma glucose, glycated hemoglobin (HbA1c), blood urea nitrogen (BUN), creatinine, uric acid, aspartate transaminase (AST), alanine transaminase, γ-glutamyl transpeptidase, alkaline phosphatase (Alk-p), total bilirubin, total protein, albumin, globulin, triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), thyroid-stimulating hormone (TSH), triiodothyronine, free thyroxine, and 25(OH)D.
2.iii. Statistical Analysis
Continuous variables with normal distributions are expressed equally means and standard deviations, whereas categorical variables are expressed equally frequencies and percentages. The t examination (commonly distributed variables) and chi-square test (categorical variables) were used to analyze the differences betwixt groups. Logistic regression analysis was performed to evaluate the independent biochemical parameters associated with serum 25(OH)D levels, and the data are expressed as odds ratios (ORs). Additionally, linear regression analysis was used to written report the association between independent biochemical parameters and serum 25(OH)D levels. All statistical analyses were performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). A p value of <0.05 was considered to exist statistically meaning.
3. Results
three.one. General Characteristics
The prevalence of vitamin D deficiency (25(OH)D < 30 ng/mL) was 66% amidst 1009 participants. In our sample, 527 participants had their serum 25(OH)D measurement during extended summer, and 482 participants had measurements during extended winter. The hateful serum 25(OH)D levels during extended summer were considerably higher than those during extended winter (37.38 ng/mL vs. 31.33 ng/mL, p < 0.05). Nonetheless, the proportion of 25(OH)D measurements conducted during extended summer was significantly higher in the vitamin D sufficient group than that in the vitamin D deficient grouping (56.7% vs. 49.9%, p < 0.05). Equally compared to individuals with serum 25(OH)D ≥ 30 ng/mL, those with vitamin D deficiency were younger and constituted a higher per centum of females (Tabular array 1). The CBC data indicated that participants with vitamin D deficiency had significantly lower serum hemoglobin (Hb), hematocrit (Hct), mean corpuscular Hb (MCH), and mean corpuscular book (MCV) only higher serum platelet levels than those without the deficiency. Moreover, biochemical analyses revealed that patients with vitamin D deficiency had lower HbA1c, BUN, creatinine, and AST levels only higher serum total protein, TG, and TSH levels than those of people with sufficient vitamin D. However, trunk weight, BMI, and serum levels of fasting plasma glucose, TC, HDL-C, and LDL-C were like between the vitamin D scarce and sufficient groups.
3.ii. Sex Differences Associated with Serum Vitamin D
As summarized in Table ii, we investigated the sex differences in the associations between vitamin D deficiency and biomarkers and found that both men and women with vitamin D deficiency had lower HDL-C levels and higher trunk weights, BMIs, platelet counts, and TG levels than those with sufficient vitamin D. Only men with vitamin D deficiency had higher serum white blood cell (WBC) counts and Alk-p levels than their counterparts with sufficient vitamin D. Moreover, vitamin D deficiency was significantly associated with lower MCV and serum MCH, Hb, Hct, BUN, AST, and TC levels in women.
3.3. Historic period Associated with Serum Vitamin D
We analyzed the association of historic period as part of the relationship between vitamin D deficiency and biomarkers. In adults aged over 65 years, vitamin D deficiency was associated with significantly higher BMI and WBC levels than sufficient vitamin D (Table 3). Past contrast, amongst individuals younger than 65 years, vitamin D deficiency was associated with lower MCV and serum MCH, Hb, Hct, BUN, creatinine, and AST levels and higher serum platelet counts and total protein, globulin, TG, and TSH levels than vitamin D sufficiency.
iii.4. Biomarkers Independently Associated with Serum Vitamin D: Age, Sexual practice, and Triglycerides
We performed logistic regression analysis and plant that only younger age (OR per year = 1.004, 95% CI, 1.029–i.059, p < 0.001), female sex (OR = 1.779, 95% CI, one.149–2.755, p < 0.01), and elevated serum TG levels (1 mg/dL, OR = 1.005, 95% CI, 1.002–1.007, p < 0.001) were contained chance factors for vitamin D deficiency. The serum vitamin D level of people over 65 years old was significantly higher than that of people nether 65 years old (32.three ± ten vs. 26.3 ± 9.4 ng/mL, p < 0.001). Women had lower vitamin D levels than men (25.0 ± 9.4 vs. 29.0 ± 9.viii ng/mL, p < 0.001). Every bit displayed in Figure one, elevated serum TG levels were significantly associated with decreased 25(OH)D levels (p < 0.05). The prevalence of vitamin D deficiency in people with TG levels > 200 mg/dL was significantly higher than that of people with TG levels < 200 mg/dL (74.half dozen% vs. 64.half dozen%, p < 0.05).
three.5. Relationship between Vitamin D and TG Levels Differs by Sex and Age
The vitamin D deficiency rate of men with TG levels >200 mg/dL was significantly higher than that of men with TG levels < 200 mg/dL (71.6% vs. 56.7%, p < 0.01) (Effigy 2A). Women with TG levels > 200 mg/dL and women with TG levels < 200 mg/dL had similar rates of vitamin D deficiency (83.9% vs. 74.one%, p = 0.22). Nosotros likewise plant that the vitamin D deficiency rate of adults nether 65 years of age was significantly higher when the TG levels were >200 mg/dL than when the TG levels were < 200 mg/dL (77.5% vs. 67.6%, p < 0.05) (Figure 2B). In contrast, the prevalence of vitamin D deficiency in people over 65 years of age with TG levels >200 mg/dL and TG levels < 200 mg/dL was similar (53.eight% vs. 44.5%, p = 0.52).
Additionally, nosotros analyzed the risk of hypertriglyceridemia for vitamin D deficiency in subgroups; nosotros found greater risks for vitamin D deficiency in men and in people aged younger than 65 years with elevated serum TG levels (male person sex, 1 mg/dL, OR = one.004, 95% CI, 1.002–1.007, p < 0.001; female sexual activity, 1 mg/dL, OR = 1.004, 95% CI, i.000–1.009, p = 0.049; age < 65 years, 1 mg/dL, OR = 1.002, 95% CI, i.000–1.005, p = 0.029; age ≥ 65 years, 1 mg/dL, OR = 1.005, 95% CI, 0.999–1.010, p = 0.099).
three.six. Vitamin D Deficiency Is Associated with Hypertriglyceridemia But among Heart-Anile Men
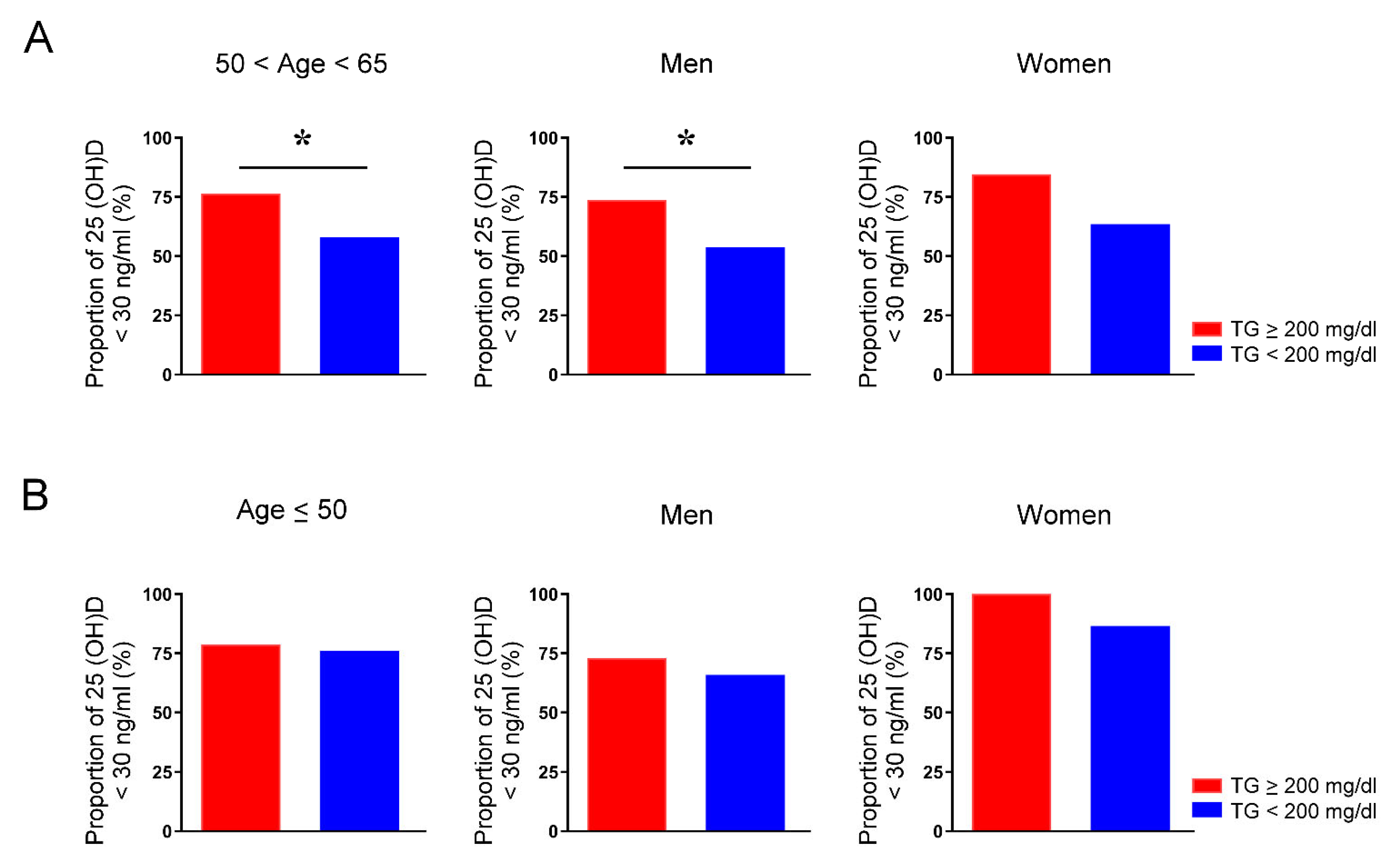
Because our results indicated that younger people are at higher take a chance of vitamin D deficiency, we analyzed the sex activity differences in the associations between vitamin D deficiency and biomarkers in participants younger than fifty years (hateful age: 41.7 years). We found that merely women with vitamin D deficiency had lower serum MCH, MCV, and creatinine but higher Alk-p levels than their counterparts with sufficient vitamin D (Table 4). Both men and women with vitamin D deficiency had lower HDL-C levels. Still, serum levels of TG were like between the vitamin D deficient and sufficient groups in men and in women. Our findings propose that vitamin D deficiency is associated with hypertriglyceridemia only among centre-aged people. Additionally, we investigated participants aged between 50 and 65 years and found that men with vitamin D deficiency had significantly higher serum TG levels than those with vitamin D sufficiency (Table v). By dissimilarity, women with and without vitamin D deficiency had similar TG levels. Moreover, the vitamin D deficiency rate of people aged fifty–65 years was significantly higher when the TG levels were >200 mg/dL than when the TG levels were < 200 mg/dL (76.4% vs. 58.1%, p < 0.05) (Effigy 3A). The vitamin D deficiency rate of men aged 50–65 years with TG levels > 200 mg/dL was significantly higher than that of men with TG levels < 200 mg/dL (73.8% vs. 53.nine%, p < 0.05). Women aged 50–65 years with TG levels greater or less than 200 mg/dL had similar rates of vitamin D deficiency (84.6% vs. 63.5%, p = 0.22). In people younger than 50 years, the vitamin D deficiency rate was similar between those with TG levels > 200 mg/dL and TG levels < 200 mg/dL (78.seven% vs. 76.one%, p = 0.74) (Figure 3B). The proportion of vitamin D deficiency in adults younger than 50 years with and without hypertriglyceridemia was similar in men (72.9% vs. 65.viii%, p = 0.twoscore) and in women (100% vs. 86.5%, p = 0.38).
4. Discussion
An assay of the National Health and Nutrition Exam Survey in the United States revealed that 14.8% of people aged older than 65 years had vitamin D deficiency and 39.9% of participants aged ≥ 20 years had vitamin D deficiency [7]. The Korea National Health and Nutrition Examination Survey revealed that vitamin D insufficiency was about prevalent between the ages of 20 and 29 years, with a prevalence of 65.0% in men and 79.ix% in women [24]. Similarly, we found that people younger than 65 years of historic period was associated with lower levels of vitamin D, contradicting the generally accepted notion that older adults tend to have more than vitamin D deficiency. One reason that older adults may be less likely to accept vitamin D deficiency is that they generally have greater health awareness and may consume healthier diets that comprise more than vitamin D. In addition, they may consume nutrient supplements that contain vitamin D; this upshot may be supported by the higher vitamin B12 levels observed in older adults with vitamin D sufficiency (information not shown). Moreover, young urban people must frequently spend considerable amounts of time indoors for work and may engage in few outdoor activities, leading to low sun exposure and a consequently higher risk of vitamin D deficiency. Our study showed that participants had college serum 25(OH)D levels during extended summer than those during extended winter, which was similar to previous findings reporting the seasonal variations in serum 25(OH)D concentrations [19,21,22]. In the present study, we also observed that women are more prone to vitamin D deficiency than men; this result is consistent with several other studies [25,26,27]. The higher prevalence of vitamin D deficiency amongst women that we discovered may be attributable to women receiving less sun exposure because lite pare is often associated with dazzler in Eastern cultures.
Several clinical studies have indicated the associations among vitamin D deficiency, anemia, and higher platelet counts [28,29,30]. Vitamin D may improve anemia through downregulating hepcidin and promoting erythropoiesis [31]. The anti-inflammatory, anti-oxidant, and anti-thrombogenic potentials of vitamin D are likewise involved in possible mechanisms for the inverse correlation of platelet counts with serum vitamin D levels [28]. In this study, we found that participants with vitamin D deficiency had significantly lower serum Hb, Hct, MCH, and MCV. However, this clan was primarily seen in women, only non in men. By dissimilarity, our study demonstrated that participants with vitamin D deficiency had college platelet counts, regardless of sex. Furthermore, the associations between vitamin D deficiency and anemia or higher platelet counts were both significant in those who were younger than 65, but not in those older than 65 years. Our findings advise that vitamin D deficiency may be included in the differential diagnosis of anemia and thrombocytosis, particularly in women or in subjects aged younger than 65 years.
Vitamin D deficiency has been linked to dyslipidemia [32,33,34]. Serum 25(OH)D levels were negatively correlated with TG and LDL-C levels, but positively correlated with HDL-C levels [32]. A meta-analysis of 41 randomized controlled trials with a total of 3434 participants revealed that vitamin D supplementation appeared to reduce serum TC, LDL-C, and TG levels [35]. However, whether sex or age affects the clan of vitamin D deficiency with dyslipidemia remains unclear. In this written report, we institute that individuals with serum 25(OH)D < 30 ng/mL had significantly higher serum TG levels than those with 25(OH)D ≥ 30 ng/mL. Withal, our data did not show a significant correlation between vitamin D levels and TC, HDL-C, or LDL-C levels, suggesting that in that location are regional or racial differences in the impact of vitamin D on lipid profiles considering vitamin D levels are strongly related to location, life patterns, and pare pigmentation. Although several biochemical parameters were presented differently between the vitamin D deficient and sufficient groups, nosotros found that only historic period, sex, and TG levels were independent risk factors for vitamin D deficiency through logistic regression analysis. Additionally, we showed that sex difference modulates the inverse association between serum TG and 25(OH)D levels. Generally, women have a higher risk of vitamin D deficiency than men. However, the negative correlation betwixt serum TG and vitamin D levels was more significant in men, and this aligns with the findings of recent studies [36,37]. Furthermore, for the get-go fourth dimension, nosotros demonstrated that the association between serum vitamin D and TG levels also differs with age. The positive clan betwixt vitamin D deficiency and hypertriglyceridemia was pregnant in those younger than 65, just non in those older than 65 years. However, subgroup analyses revealed that vitamin D deficiency is associated with hypertriglyceridemia only among men (but non women) aged between l and 65 years. A randomized controlled trial showed that supplementation with vitamin D (200 IU/24-hour interval for sixteen weeks) did not change serum lipid levels in women aged 24.7 years [38]. Appropriately, centre-aged men with hypertriglyceridemia may exist associated with a high possibility of vitamin D deficiency.
Eating habits have a great bear upon on TG metabolism. Higher intake of uncomplicated sugars was institute to contribute to increased plasma concentrations of TG [39]. A recent analysis of 191,558 participants revealed that higher fish consumption was associated with lower TG levels [40]. Replacing elementary carbohydrates with complex carbohydrates and taking omega-3 fish oil are both effective treatments for hypertriglyceridemia [41]. In add-on, higher fish consumption has been demonstrated to increase serum 25(OH)D levels significantly amidst 402 healthy people [42]. Therefore, vitamin D may mediate the TG lowering effect of fish oil, at least in part. Rats with loftier-saccharide-diet-induced obesity exhibited higher TC, TG, and LDL-C as well as lower HDL-C levels than control rats. All of these lipid alterations were attenuated by vitamin D supplementation [43]. Vitamin D significantly reduced plasma TG and LDL-C levels in mice with high-fat diets and streptozotocin-induced diabetes mellitus [44]. Furthermore, vitamin D alleviated the increases in plasma TC, TG, and LDL-C levels and the decrease in HDL-C levels caused by ovariectomy in female rats [45]. Treatment with calcitriol, the bioactive vitamin D metabolite, decreased plasma concentrations of TG and LDL-C and increased HDL-C levels in hypercholesterolemic male person rabbits [46]. In addition, sterol regulatory element-bounden proteins (SREBPs) are transcription factors that modulate the lipid metabolism by upregulating the expression of lipogenic genes. Finally, 25(OH)D has been demonstrated to impair SREBP activation by inducing its proteolytic degradation [47]. These findings suggest the therapeutic potential of vitamin D for hyperlipidemia.
This study has several limitations. First, this is a cross-sectional report; thus, causality cannot exist proven. 2nd, this study was conducted using data from patients at a unmarried centre, and the generalizability of the results to the entire Taiwanese population is limited given that the sample sizes within our subgroups were small. Third, nosotros were unable to obtain data on many misreckoning factors that may influence serum vitamin D and TG levels, including medical history, smoking, dietary characteristics, sun exposure, physical activity levels, and work environment. More piece of work is required to clarify whether the correction of vitamin D deficiency can yield clinically meaningful changes in lipid concentrations and, thereby, further reduce the risk of cardiovascular diseases.
v. Conclusions
Adults younger than 65 years old, women, and middle-aged men with high serum TG levels might have a higher risk of vitamin D deficiency.
Author Contributions
Conceptualization, T.-W.L. and Y.-J.C.; Methodology, T.-W.L., Y.-H.K. and Y.-J.C.; Software, Y.-L.C. and C.-Y.Due west.; Validation, T.-I.L. and C.-Y.Due west.; Formal Analysis, Y.-L.C. and T.-W.L.; Data Curation, T.-I.50. and C.-Y.West.; Writing—Original Typhoon Preparation, Y.-L.C. and T.-W.L.; Supervision, Y.-H.Chiliad. and Y.-J.C.; Funding Acquisition, Y.-H.K. and T.-Westward.Fifty. All authors accept read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Ministry of Science and Engineering of Taiwan (Virtually 107-2314-B-038-099-MY3), and Wan Fang Hospital, Taipei Medical Academy (107-wf-eva-04). The funding sources had no involvement in the study design; in the collection, analysis and inter-pretation of data; in the writing of the manuscript; and in the decision to submit the article for publication.
Institutional Review Board Statement
The written report was conducted in accordance with the Declaration of Helsinki, and approved by the Taipei Medical University Joint Institutional Review Board (protocol code: N202006010).
Informed Consent Argument
Informed consent was waived considering of the retrospective nature of the written report and the analysis used anonymous clinical data.
Data Availability Statement
The data presented in this written report are available on asking from the corresponding author. The data are not publicly bachelor due to their containing data that could compromise the privacy of inquiry participants.
Conflicts of Interest
The authors declare no conflict of involvement.
References
- Amrein, Chiliad.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, Chiliad.; Pilz, Due south.; Malle, O. Vitamin D deficiency ii.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.West.; Kao, Y.H.; Chen, Y.J.; Chao, T.F.; Lee, T.I. Therapeutic potential of vitamin D in AGE/RAGE-related cardiovascular diseases. Jail cell. Mol. Life Sci. 2019, 76, 4103–4115. [Google Scholar] [CrossRef]
- Lee, T.Westward.; Lee, T.I.; Chang, C.J.; Lien, G.Southward.; Kao, Y.H.; Chao, T.F.; Chen, Y.J. Potential of vitamin D in treating diabetic cardiomyopathy. Nutr. Res. 2015, 35, 269–279. [Google Scholar] [CrossRef]
- Jorde, R.; Grimnes, G. Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Prog. Lipid Res. 2011, l, 303–312. [Google Scholar] [CrossRef]
- Parva, N.R.; Tadepalli, S.; Singh, P.; Qian, A.; Joshi, R.; Kandala, H.; Nookala, V.K.; Cheriyath, P. Prevalence of Vitamin D Deficiency and Associated Run a risk Factors in the US Population (2011–2012). Cureus 2018, 10, e2741. [Google Scholar] [CrossRef]
- Theodoratou, E.; Tzoulaki, I.; Zgaga, L.; Ioannidis, J.P. Vitamin D and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014, 348, g2035. [Google Scholar] [CrossRef]
- Zhen, D.; Liu, L.; Guan, C.; Zhao, N.; Tang, X. High prevalence of vitamin D deficiency among middle-anile and elderly individuals in northwestern Cathay: Its relationship to osteoporosis and lifestyle factors. Os 2015, 71, 1–vi. [Google Scholar] [CrossRef]
- Chailurkit, Fifty.O.; Aekplakorn, Westward.; Ongphiphadhanakul, B. Regional variation and determinants of vitamin D status in sunshine-abundant Thailand. BMC Public Wellness 2011, 11, 853. [Google Scholar] [CrossRef]
- Jang, H.; Koo, F.K.; Ke, L.; Clemson, L.; Cant, R.; Fraser, D.R.; Seibel, Chiliad.J.; Tseng, M.; Mpofu, E.; Stonemason, R.S.; et al. Culture and lord's day exposure in immigrant E Asian women living in Australia. Women Health 2013, 53, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Garbutcheon-Singh, K.B.; Dixit, S.; Brown, P.; Smith, S.D. The influence of age and gender in noesis, behaviors and attitudes towards sun protection: A cantankerous-sectional survey of Australian outpatient clinic attendees. Am. J. Clin. Dermatol. 2015, 16, 47–54. [Google Scholar] [CrossRef] [PubMed]
- MacLaughlin, J.; Holick, M.F. Crumbling decreases the capacity of human peel to produce vitamin D3. J. Clin. Investig. 1985, 76, 1536–1538. [Google Scholar] [CrossRef]
- Wyskida, M.; Wieczorowska-Tobis, K.; Chudek, J. Prevalence and factors promoting the occurrence of vitamin D deficiency in the elderly. Postep. Hig. Med. Dosw. 2017, 71, 198–204. [Google Scholar] [CrossRef]
- Chalcraft, J.R.; Cardinal, L.M.; Wechsler, P.J.; Hollis, B.W.; Gerow, Thou.1000.; Alexander, B.K.; Keith, J.F.; Larson-Meyer, D.E. Vitamin D Synthesis Following a Single Tour of Sun Exposure in Older and Younger Men and Women. Nutrients 2020, 12, 2237. [Google Scholar] [CrossRef]
- Fan, P.; Wang, Q.; Li, J.; Lu, C.; Xu, Y.; Cao, H.; Xie, X.; Wu, X.; Guo, Y.; Liu, T.; et al. Poor Condition of Vitamin D: A Survey of Area with Lowest Sunlight Radiation in Sichuan, China. Front. Endocrinol. 2021, 12, 626983. [Google Scholar] [CrossRef]
- Yu, H.J.; Kwon, M.J.; Woo, H.Y.; Park, H. Assay of 25-Hydroxyvitamin D Status According to Age, Gender, and Seasonal Variation. J. Clin. Lab. Anal. 2016, 30, 905–911. [Google Scholar] [CrossRef]
- Andersen, S.; Jakobsen, A.; Laurberg, P. Vitamin D status in Northward Greenland is influenced by diet and season: Indicators of dermal 25-hydroxy vitamin D product n of the Arctic Circle. Br. J. Nutr. 2013, 110, 50–57. [Google Scholar] [CrossRef]
- Papadakis, G.; Keramidas, I.; Kakava, K.; Pappa, T.; Villiotou, V.; Triantafillou, E.; Drosou, A.; Tertipi, A.; Kaltzidou, 5.; Pappas, A. Seasonal variation of serum vitamin D amidst Greek female person patients with osteoporosis. Vivo 2015, 29, 409–413. [Google Scholar]
- Gocho, Y.; Tanaka, M.; Sugawara, H.; Furuhashi, 1000.; Moniwa, N.; Yamashita, T.; Takizawa, H.; Mukai, H.; Ohno, Chiliad.; Maeda, T.; et al. Seasonal variation of serum 25-hydroxyvitamin D level in hemodialysis patients in the northernmost island of Japan. Clin. Exp. Nephrol. 2021, 25, 1360–1366. [Google Scholar] [CrossRef]
- Andersen, R.; Brot, C.; Jakobsen, J.; Mejborn, H.; Mølgaard, C.; Skovgaard, L.T.; Trolle, Eastward.; Tetens, I.; Ovesen, Fifty. Seasonal changes in vitamin D condition amongst Danish adolescent girls and elderly women: The influence of sun exposure and vitamin D intake. Eur. J. Clin. Nutr. 2013, 67, 270–274. [Google Scholar] [CrossRef]
- Huta-Osiecka, A.; Wochna, Thou.; Kasprzak, Z.; Nowak, A. Seasonal variation of 25-Hydroxyvitamin D and indices of carbohydrate and lipid metabolism in postmenopausal women. PeerJ 2021, 9, e11341. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-C.; Yen, Due west.-L.; Liu, C.-W. Solar Global Radiation Data of Taiwan in Terms of the Typical Meteorological Year betwixt 2004 and 2013. J. Taiwan Free energy 2016, 3, 89–101. [Google Scholar]
- Choi, H.S.; Oh, H.J.; Choi, H.; Choi, W.H.; Kim, J.G.; Kim, K.M.; Kim, K.J.; Rhee, Y.; Lim, Southward.Thou. Vitamin D insufficiency in Korea--a greater threat to younger generation: The Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin. Endocrinol. Metab. 2011, 96, 643–651. [Google Scholar] [CrossRef]
- AlQuaiz, A.M.; Kazi, A.; Fouda, M.; Alyousefi, North. Age and gender differences in the prevalence and correlates of vitamin D deficiency. Arch. Osteoporos. 2018, thirteen, 49. [Google Scholar] [CrossRef]
- Muscogiuri, Yard.; Barrea, 50.; Somma, C.D.; Laudisio, D.; Salzano, C.; Pugliese, G.; de Alteriis, G.; Colao, A.; Savastano, South. Sex Differences of Vitamin D Condition across BMI Classes: An Observational Prospective Accomplice Study. Nutrients 2019, 11, 3034. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, N.; Cheng, South.; Wang, Z.; Qin, Y. Gender Differences in Vitamin D Status in China. Med. Sci. Monit. 2019, 25, 7094–7099. [Google Scholar] [CrossRef]
- Park, Y.C.; Kim, J.; Seo, M.Southward.; Hong, S.W.; Cho, East.S.; Kim, J.Chiliad. Inverse relationship between vitamin D levels and platelet indices in Korean adults. Hematology 2017, 22, 623–629. [Google Scholar] [CrossRef]
- Perlstein, T.S.; Pande, R.; Berliner, N.; Vanasse, One thousand.J. Prevalence of 25-hydroxyvitamin D deficiency in subgroups of elderly persons with anemia: Association with anemia of inflammation. Blood 2011, 117, 2800–2806. [Google Scholar] [CrossRef]
- Sim, J.J.; Lac, P.T.; Liu, I.50.; Meguerditchian, Southward.O.; Kumar, V.A.; Kujubu, D.A.; Rasgon, S.A. Vitamin D deficiency and anemia: A cross-sectional written report. Ann. Hematol. 2010, 89, 447–452. [Google Scholar] [CrossRef]
- Smith, E.K.; Tangpricha, V. Vitamin D and anemia: Insights into an emerging association. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Ten.; Peng, G.; Chen, Due south.; Wu, S.; Zhang, W. Vitamin D deficiency is associated with dyslipidemia: A cantankerous-sectional study in 3788 subjects. Curr. Med. Res. Opin. 2019, 35, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Figenschau, Y.; Hutchinson, M.; Emaus, N.; Grimnes, G. High serum 25-hydroxyvitamin D concentrations are associated with a favorable serum lipid profile. Eur. J. Clin. Nutr. 2010, 64, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Yang, 1000.; Liu, J.; Fu, S.; Tang, 10.; Ma, L.; Sun, W.; Niu, Y.; Jing, G.; Niu, Q. Vitamin D Status and Correlation with Glucose and Lipid Metabolism in Gansu Province, China. Diabetes Metab. Syndr. Obes. 2020, 13, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Dibaba, D.T. Issue of vitamin D supplementation on serum lipid profiles: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Liu, Q.; Zhang, Q.; Wan, Z.; Hu, L.; Xu, R.; Cheng, A.; Lv, Y.; Wang, L. Sex-Specific Clan between Serum Vitamin D Status and Lipid Profiles: A Cross-Exclusive Study of a Heart-Anile and Elderly Chinese Population. J. Nutr. Sci. Vitaminol. 2020, 66, 105–113. [Google Scholar] [CrossRef]
- Wang, Y.; Si, S.; Liu, J.; Wang, Z.; Jia, H.; Feng, G.; Sun, L.; Song, Southward.J. The Associations of Serum Lipids with Vitamin D Status. PLoS 1 2016, eleven, e0165157. [Google Scholar] [CrossRef]
- Toxqui, L.; Blanco-Rojo, R.; Wright, I.; Pérez-Granados, A.M.; Vaquero, 1000.P. Changes in blood pressure and lipid levels in immature women consuming a vitamin D-fortified skimmed milk: A randomised controlled trial. Nutrients 2013, 5, 4966–4977. [Google Scholar] [CrossRef]
- Busnatu, S.S.; Salmen, T.; Pana, M.A.; Rizzo, M.; Stallone, T.; Papanas, N.; Popovic, D.; Tanasescu, D.; Serban, D.; Stoian, A.P. The Role of Fructose as a Cardiovascular Gamble Gene: An Update. Metabolites 2022, 12, 67. [Google Scholar] [CrossRef]
- Mohan, D.; Mente, A.; Dehghan, M.; Rangarajan, S.; O'Donnell, M.; Hu, W.; Dagenais, K.; Wielgosz, A.; Lear, Due south.; Wei, Fifty.; et al. Associations of Fish Consumption with Risk of Cardiovascular Disease and Mortality Amid Individuals with or without Vascular Disease from 58 Countries. JAMA Intern. Med. 2021, 181, 631–649. [Google Scholar] [CrossRef]
- Subramanian, Southward. Approach to the Patient with Moderate Hypertriglyceridemia. J. Clin. Endocrinol. Metab. 2022, dgac085, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, K.; Muscogiuri, G.; Paganitsa, Thousand.; Parvouleskou, G.; Syriou, 5.; Karagkoynis, P.; Stratigou, T.; Vallianou, Northward.; Christodoulatos, G.South.; Karampela, I.; et al. Adherence to the Mediterranean diet is an independent predictor of circulating vitamin D levels in normal weight and non-smoker adults: An observational cross-sectional written report. Int. J. Food Sci. Nutr. 2021, 72, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Bima, A.I.; Mahdi, A.S.; Al Fayez, F.F.; Khawaja, T.Grand.; Abo El-Khair, Southward.M.; Elsamanoudy, A.Z. Cellular Senescence and Vitamin D Deficiency Play a Function in the Pathogenesis of Obesity-Associated Subclinical Atherosclerosis: Study of the Potential Protective Role of Vitamin D Supplementation. Cells 2021, 10, 920. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, H.; Lim, Y. Vitamin D(three) improves lipophagy-associated renal lipid metabolism and tissue damage in diabetic mice. Nutr. Res. 2020, 80, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Babaei, P.; Damirchi, A.; Hoseini, R. The interaction effects of aerobic exercise preparation and vitamin D supplementation on plasma lipid profiles and insulin resistance in ovariectomized rats. J. Exerc. Nutr. Biochem. 2015, 19, 173–182. [Google Scholar] [CrossRef]
- Radagdam, S.; Asoudeh-Fard, A.; Karimi, One thousand.A.; Faridvand, Y.; Gholinejad, Z.; Gerayesh Nejad, S. Calcitriol modulates cholesteryl ester transfer protein (CETP) levels and lipid contour in hypercholesterolemic male rabbits: A pilot written report. Int. J. Vitam. Nutr. Res. 2021, 91, 212–216. [Google Scholar] [CrossRef]
- Asano, L.; Watanabe, M.; Ryoden, Y.; Usuda, K.; Yamaguchi, T.; Khambu, B.; Takashima, Chiliad.; Sato, S.I.; Sakai, J.; Nagasawa, Chiliad.; et al. Vitamin D Metabolite, 25-Hydroxyvitamin D, Regulates Lipid Metabolism by Inducing Degradation of SREBP/SCAP. Cell Chem. Biol. 2017, 24, 207–217. [Google Scholar] [CrossRef]
Figure 1. Correlations between serum levels of 25-hydroxyvitamin D (25(OH)D) and triglyceride (TG) levels in total study population. Increased serum TG levels were significantly associated with decreased 25(OH)D levels.
Figure ane. Correlations betwixt serum levels of 25-hydroxyvitamin D (25(OH)D) and triglyceride (TG) levels in full written report population. Increased serum TG levels were significantly associated with decreased 25(OH)D levels.
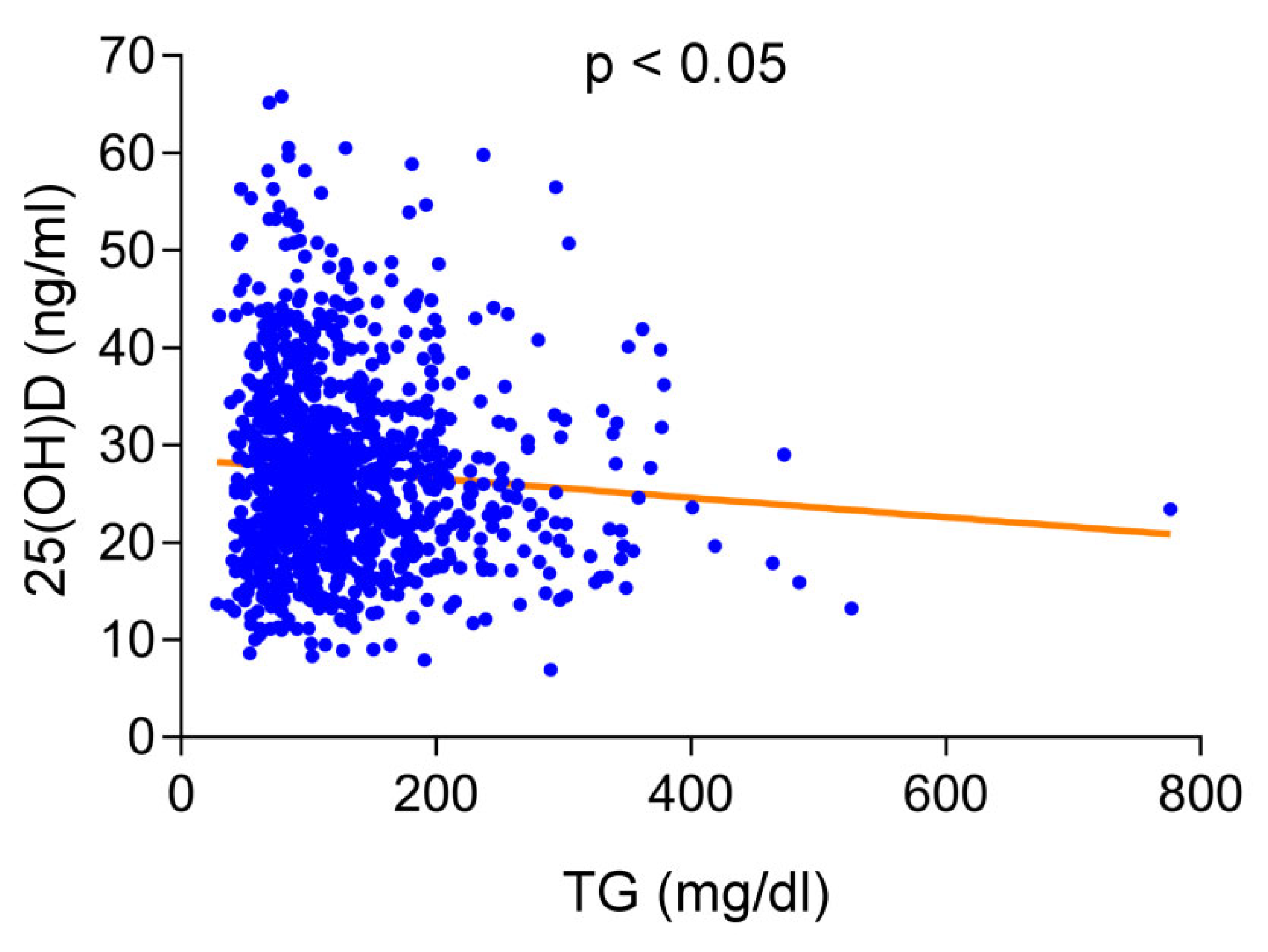
Figure two. Proportion of vitamin D deficiency in people with and without hypertriglyceridemia is modulated by sex and age. The rate of serum 25-hydroxyvitamin D (25(OH)D) levels below 30 ng/mL in (A) men and women and (B) individuals aged younger and older than 65 years whose serum levels of triglycerides (TG) were greater or less than 200 mg/dL. * p < 0.05, ** p < 0.01.
Effigy 2. Proportion of vitamin D deficiency in people with and without hypertriglyceridemia is modulated by sex and age. The charge per unit of serum 25-hydroxyvitamin D (25(OH)D) levels below 30 ng/mL in (A) men and women and (B) individuals aged younger and older than 65 years whose serum levels of triglycerides (TG) were greater or less than 200 mg/dL. * p < 0.05, ** p < 0.01.
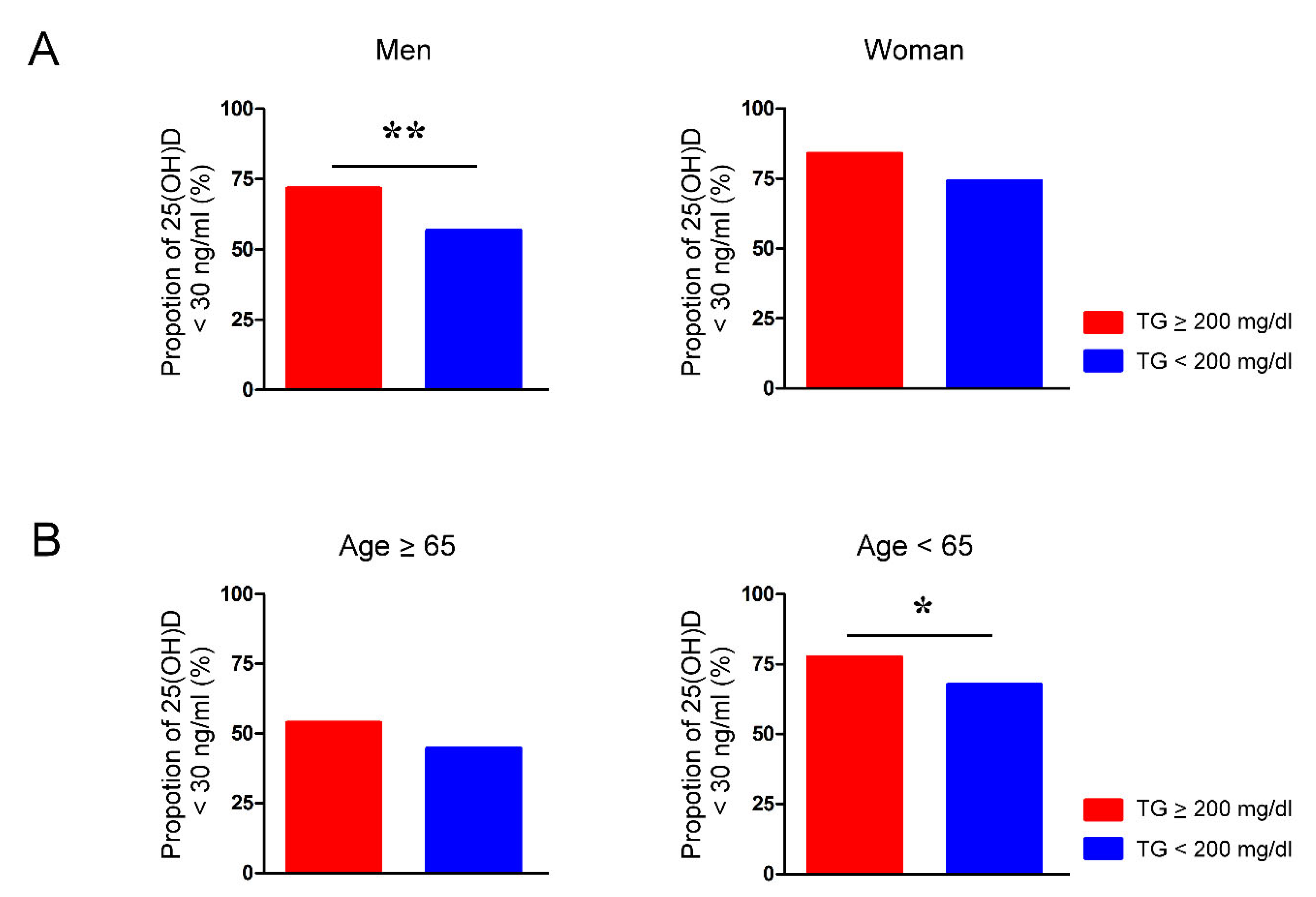
Figure iii. Proportion of vitamin D deficiency in people aged between 50 and 65 years or younger than 50 years with and without hypertriglyceridemia. The charge per unit of serum 25-hydroxyvitamin D (25(OH)D) levels below 30 ng/mL of individuals in total, men, and women aged between l and 65 years (A) and younger than l years (B) whose serum levels of triglycerides (TG) were greater or less than 200 mg/dL. * p < 0.05.
Figure 3. Proportion of vitamin D deficiency in people aged between 50 and 65 years or younger than 50 years with and without hypertriglyceridemia. The rate of serum 25-hydroxyvitamin D (25(OH)D) levels below 30 ng/mL of individuals in full, men, and women aged between 50 and 65 years (A) and younger than l years (B) whose serum levels of triglycerides (TG) were greater or less than 200 mg/dL. * p < 0.05.
Tabular array 1. Associations between vitamin D condition and biomarkers in all participants.
Tabular array one. Associations between vitamin D status and biomarkers in all participants.
| Vitamin D < thirty ng/mL (n = 665) | Vitamin D ≥ thirty ng/mL (n = 344) | p Value | |
|---|---|---|---|
| Age (years) | 50.02 ± x.99 | 55.62 ± xi.65 | <0.001 |
| Female (%) | 326 (49) | 110 (32) | <0.001 |
| BW (kg) | 67.47 ± fourteen.21 | 67.39 ± 12.89 | 0.93 |
| BMI (kg/m2) | 24.48 ± 3.87 | 24.28 ± 3.39 | 0.39 |
| WBC (103/μL) | half dozen.21 ± 1.73 | half dozen.01 ± one.65 | 0.07 |
| RBC (10six/μL) | iv.89 ± 0.51 | four.92 ± 0.52 | 0.37 |
| Hb (g/dL) | 14.56 ± i.58 | 14.93 ± ane.33 | <0.001 |
| Hct (%) | 43.14 ± 4.25 | 44.nineteen ± 3.60 | <0.001 |
| MCH (pg) | 29.89 ± 2.90 | 30.51 ± two.58 | <0.001 |
| MCV (fL) | 88.52 ± seven.xvi | 90.22 ± half dozen.52 | <0.001 |
| MCHC (k/dL) | 33.71 ± 0.95 | 33.78 ± 0.84 | 0.25 |
| Platelets (10three/μL) | 247.1 ± 59.xviii | 230.5 ± 54.03 | <0.001 |
| MPV (fL) | 8.46 ± 0.89 | 8.50 ± 0.88 | 0.48 |
| Fasting glucose (mg/dL) | 96.85 ± 22.96 | 99.13 ± 22.52 | 0.thirteen |
| HbA1c (%) | 5.63 ± 0.78 | 5.74 ± 0.88 | <0.05 |
| BUN (mg/dL) | 10.49 ± 3.55 | xi.37 ± 3.55 | <0.001 |
| Creatinine (mg/dL) | 0.80 ± 0.24 | 0.85 ± 0.eighteen | <0.005 |
| Uric acrid (mg/dL) | 5.80 ± 1.42 | 5.95 ± 1.38 | 0.xi |
| AST (U/L) | twenty.70 ± 8.48 | 22.26 ± 8.91 | <0.01 |
| ALT (U/L) | 23.31 ± 16.77 | 24.01 ± sixteen.97 | 0.53 |
| r-GT (U/L) | 25.55 ± 26.28 | 27.14 ± 24.ninety | 0.35 |
| Alk-p (U/L) | 58.73 ± xviii.61 | 57.38 ± 15.31 | 0.22 |
| Total bilirubin (mg/dL) | i.03 ± 0.45 | 1.07 ± 0.42 | 0.xviii |
| Full protein (g/dL) | 7.xv ± 0.39 | seven.09 ± 0.40 | <0.05 |
| Albumin (g/dL) | 4.46 ± 0.25 | four.44 ± 0.25 | 0.16 |
| Globulin (g/dL) | 2.69 ± 0.34 | ii.65 ± 0.36 | 0.10 |
| TG (mg/dL) | 133.2 ± 76.04 | 119.9 ± 64.28 | <0.005 |
| Total cholesterol (mg/dL) | 192.eight ± 39.05 | 193.1 ± 38.21 | 0.91 |
| HDL-C (mg/dL) | 51.26 ± 14.17 | 52.38 ± 14.sixty | 0.24 |
| LDL-C (mg/dL) | 124.four ± 34.06 | 125.4 ± 34.38 | 0.66 |
| TSH (μIU/mL) | 2.36 ± 3.fifty | ii.03 ± 1.42 | <0.05 |
| T3 (ng/dL) | 101.nine ± eighteen.76 | 102.7 ± 17.36 | 0.50 |
| Gratuitous T4 (ng/dL) | 0.94 ± 0.23 | 0.92 ± 0.13 | 0.nineteen |
Tabular array ii. Associations between vitamin D status and biomarkers in men and women.
Tabular array two. Associations between vitamin D condition and biomarkers in men and women.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Vitamin D < 30 (n = 339) | Vitamin D ≥ 30 (n = 234) | p Value | Vitamin D < thirty (northward = 326) | Vitamin D ≥ thirty (n = 110) | p Value | |
| Age (years) | 50.30 ± x.95 | 55.07 ± 11.91 | <0.001 | 49.72 ± xi.04 | 56.77 ± 11.06 | <0.001 |
| BW (kg) | 76.21 ± 12.33 | 72.eighty ± xi.23 | <0.001 | 58.38 ± ix.59 | 55.ninety ± seven.58 | <0.01 |
| BMI (kg/mii) | 25.86 ± 3.64 | 25.18 ± 3.29 | <0.05 | 23.05 ± 3.57 | 22.36 ± 2.76 | <0.05 |
| WBC (x3/μL) | half-dozen.52 ± i.70 | vi.21 ± 1.62 | <0.05 | five.88 ± 1.70 | v.58 ± 1.65 | 0.10 |
| RBC (x6/μL) | 5.12 ± 0.fifty | 5.09 ± 0.48 | 0.38 | 4.64 ± 0.40 | four.56 ± 0.40 | 0.06 |
| Hb (chiliad/dL) | 15.42 ± 1.thirty | 15.41 ± 1.22 | 0.95 | thirteen.66 ± 1.33 | xiii.91 ± 0.91 | <0.05 |
| Hct (%) | 45.52 ± 3.56 | 45.54 ± 3.18 | 0.93 | 40.66 ± iii.42 | 41.xxx ± 2.63 | <0.05 |
| MCH (pg) | 30.22 ± two.64 | 30.46 ± 2.86 | 0.29 | 29.55 ± 3.11 | 30.61 ± ane.88 | <0.001 |
| MCV (fL) | 89.15 ± vi.67 | 89.95 ± 7.16 | 0.17 | 87.87 ± 7.59 | 90.81 ± 4.86 | <0.001 |
| MCHC (m/dL) | 33.86 ± 0.86 | 33.82 ± 0.90 | 0.63 | 33.56 ± 1.02 | 33.69 ± 0.69 | 0.13 |
| Platelets (10three/μL) | 236.five ± sixty.34 | 224.0 ± 48.86 | <0.01 | 258.0 ± 55.94 | 244.2 ± 61.98 | <0.05 |
| MPV (fL) | 8.45 ± 0.87 | 8.50 ± 0.88 | 0.52 | viii.47 ± 0.91 | eight.50 ± 0.89 | 0.71 |
| Fasting glucose (mg/dL) | 100.8 ± 24.49 | 100.7 ± 23.35 | 0.97 | 92.76 ± 20.52 | 95.78 ± twenty.36 | 0.18 |
| HbA1c (%) | five.74 ± 0.91 | v.81 ± 0.97 | 0.42 | 5.51 ± 0.61 | 5.62 ± 0.65 | 0.xiv |
| BUN (mg/dL) | eleven.54 ± 3.68 | 11.95 ± 3.44 | 0.18 | 9.forty ± 3.05 | x.14 ± 3.49 | <0.05 |
| Creatinine (mg/dL) | 0.95 ± 0.25 | 0.93 ± 0.fourteen | 0.35 | 0.65 ± 0.09 | 0.67 ± 0.10 | 0.14 |
| Uric acid (mg/dL) | 6.57 ± 1.28 | half-dozen.42 ± i.27 | 0.19 | 5.01 ± ane.09 | 4.94 ± 1.03 | 0.55 |
| AST (U/Fifty) | 22.18 ± 9.68 | 22.83 ± nine.xl | 0.42 | 19.15 ± half dozen.70 | 21.04 ± seven.65 | <0.05 |
| ALT (U/50) | 28.41 ± nineteen.22 | 25.92 ± 17.73 | 0.xi | 18.02 ± 11.63 | 19.95 ± fourteen.48 | 0.20 |
| r-GT (U/L) | 31.75 ± 32.72 | 30.79 ± 28.xv | 0.70 | 19.xi ± 14.67 | 19.32 ± 12.77 | 0.89 |
| Alk-p (U/L) | sixty.02 ± nineteen.54 | 57.00 ± 14.38 | <0.05 | 57.39 ± 17.52 | 58.20 ± 17.15 | 0.67 |
| Total bilirubin (mg/dL) | 1.13 ± 0.49 | 1.13 ± 0.43 | 0.99 | 0.92 ± 0.37 | 0.94 ± 0.36 | 0.75 |
| Total protein (k/dL) | 7.12 ± 0.38 | 7.08 ± 0.38 | 0.xx | 7.19 ± 0.twoscore | 7.12 ± 0.43 | 0.12 |
| Albumin (g/dL) | four.51 ± 0.26 | 4.46 ± 0.26 | 0.06 | 4.41 ± 0.24 | 4.38 ± 0.22 | 0.23 |
| Globulin (g/dL) | 2.61 ± 0.33 | 2.61 ± 0.35 | 0.95 | ii.77 ± 0.34 | ii.73 ± 0.36 | 0.29 |
| TG (mg/dL) | 152.i ± 83.64 | 128.eight ± seventy.04 | <0.001 | 113.6 ± 61.47 | 101.0 ± 44.50 | <0.05 |
| Total cholesterol (mg/dL) | 187.6 ± 36.89 | 186.viii ± 38.65 | 0.78 | 198.ii ± 40.53 | 206.6 ± 33.63 | <0.05 |
| HDL-C (mg/dL) | 44.xvi ± 9.62 | 47.48 ± 11.46 | <0.001 | 58.65 ± 14.38 | 62.fourscore ± fifteen.fourteen | <0.05 |
| LDL-C (mg/dL) | 124.8 ± 32.88 | 123.eight ± 36.08 | 0.72 | 124.1 ± 35.29 | 128.9 ± 30.29 | 0.19 |
| TSH (μIU/mL) | two.08 ± 1.forty | 2.01 ± i.48 | 0.57 | ii.64 ± four.79 | 2.08 ± 1.30 | 0.06 |
| T3 (ng/dL) | 104.viii ± 18.85 | 103.two ± 16.12 | 0.xxx | 98.9 ± eighteen.23 | 101.5 ± nineteen.77 | 0.xx |
| Free T4 (ng/dL) | 0.96 ± 0.27 | 0.93 ± 0.xiii | 0.14 | 0.92 ± 0.17 | 0.90 ± 0.xi | 0.34 |
Table iii. Associations between vitamin D status and biomarkers in individuals aged older and younger than 65 years.
Tabular array 3. Associations between vitamin D status and biomarkers in individuals anile older and younger than 65 years.
| Age ≥ 65 Years | Historic period < 65 Years | |||||
|---|---|---|---|---|---|---|
| Vitamin D < 30 (n = 60) | Vitamin D ≥ 30 (northward = 72) | p Value | Vitamin D < thirty (n = 605) | Vitamin D ≥ 30 (north = 272) | p Value | |
| Female (%) | 28 (46.67) | 23 (31.94) | 0.08 | 298 (49.26) | 87 (31.99) | <0.001 |
| BW (kg) | 63.76 ± 11.xvi | 62.53 ± 12.12 | 0.54 | 67.84 ± xiv.43 | 68.68 ± 12.80 | 0.38 |
| BMI (kg/mtwo) | 24.86 ± 3.22 | 23.68 ± 3.44 | <0.05 | 24.44 ± 0.92 | 24.44 ± 3.37 | 0.98 |
| WBC (103/μL) | half dozen.20 ± 1.65 | 5.66 ± 1.29 | <0.05 | 6.21 ± 1.74 | 6.10 ± 1.72 | 0.38 |
| RBC (ten6/μL) | 4.65 ± 0.47 | 4.76 ± 0.51 | 0.20 | 4.91 ± 0.51 | iv.96 ± 0.52 | 0.20 |
| Hb (yard/dL) | fourteen.40 ± i.32 | fourteen.62 ± ane.25 | 0.32 | 14.57 ± 1.60 | 15.01 ± i.34 | <0.001 |
| Hct (%) | 42.55 ± 3.74 | 43.24 ± 3.37 | 0.26 | 43.19 ± four.29 | 44.44 ± 3.63 | <0.001 |
| MCH (pg) | 31.02 ± two.07 | xxx.85 ± 2.59 | 0.67 | 29.78 ± 2.94 | 30.42 ± 2.58 | <0.005 |
| MCV (fL) | 91.60 ± 5.04 | 91.xix ± half dozen.79 | 0.69 | 88.22 ± 7.27 | 89.97 ± 6.43 | <0.001 |
| MCHC (g/dL) | 33.85 ± 0.76 | 33.81 ± 0.73 | 0.75 | 33.70 ± 0.97 | 33.77 ± 0.87 | 0.28 |
| Platelets (ten3/μL) | 221.6 ± 45.85 | 215.3 ± 47.09 | 0.43 | 249.6 ± 59.79 | 234.5 ± 55.10 | <0.001 |
| MPV (fL) | viii.66 ± 0.85 | viii.61 ± 0.92 | 0.72 | viii.44 ± 0.89 | 8.47 ± 0.87 | 0.60 |
| Fasting glucose (mg/dL) | 105.eight ± 25.36 | 106.8 ± 28.55 | 0.83 | 95.97 ± 22.54 | 97.12 ± 20.22 | 0.45 |
| HbA1c (%) | 5.96 ± 0.86 | 6.14 ± one.28 | 0.32 | 5.sixty ± 0.77 | v.64 ± 0.71 | 0.45 |
| BUN (mg/dL) | 12.56 ± 5.04 | xiii.25 ± 3.74 | 0.38 | 10.29 ± iii.30 | 10.87 ± 3.34 | <0.05 |
| Creatinine (mg/dL) | 0.86 ± 0.48 | 0.85 ± 0.19 | 0.94 | 0.80 ± 0.20 | 0.85 ± 0.17 | <0.001 |
| Uric acid (mg/dL) | five.92 ± 1.fifty | 5.84 ± i.35 | 0.74 | 5.79 ± 1.42 | 5.98 ± 1.39 | 0.06 |
| AST (U/50) | 22.38 ± nine.46 | 22.thirteen ± 5.74 | 0.86 | xx.53 ± 8.37 | 22.29 ± 9.58 | <0.01 |
| ALT (U/L) | 20.86 ± 12.twoscore | xx.54 ± 7.83 | 0.86 | 23.55 ± 17.13 | 24.93 ± eighteen.56 | 0.28 |
| r-GT (U/L) | 27.65 ± forty.88 | 25.69 ± 20.97 | 0.73 | 25.34 ± 24.40 | 27.53 ± 25.86 | 0.23 |
| Alk-p (U/Fifty) | 63.01 ± 17.45 | 61.37 ± 15.82 | 0.57 | 58.30 ± xviii.69 | 56.33 ± fifteen.02 | 0.09 |
| Total bilirubin (mg/dL) | 0.96 ± 0.31 | one.03 ± 0.44 | 0.26 | one.04 ± 0.46 | i.08 ± 0.42 | 0.21 |
| Total poly peptide (g/dL) | vii.08 ± 0.40 | vii.05 ± 0.46 | 0.77 | 7.16 ± 0.39 | 7.10 ± 0.30 | <0.05 |
| Albumin (g/dL) | 4.33 ± 0.25 | 4.31 ± 0.25 | 0.54 | iv.47 ± 0.25 | iv.47 ± 0.23 | 0.92 |
| Globulin (g/dL) | two.74 ± 0.38 | 2.74 ± 0.42 | 0.94 | ii.68 ± 0.34 | 2.62 ± 0.34 | <0.05 |
| TG (mg/dL) | 134.iv ± 69.92 | 114.2 ± 65.68 | 0.08 | 133.1 ± 76.68 | 121.5 ± 63.94 | <0.05 |
| Total cholesterol (mg/dL) | 192.ane ± 41.31 | 186.5 ± 36.71 | 0.40 | 129.nine ± 38.85 | 194.8 ± 38.47 | 0.48 |
| HDL-C (mg/dL) | 51.03 ± xiii.09 | fifty.91 ± 13.40 | 0.96 | 51.29 ± 14.28 | 52.77 ± xiv.90 | 0.16 |
| LDL-C (mg/dL) | 121.9 ± twoscore.36 | 120.5 ± 34.92 | 0.83 | 124.7 ± 33.33 | 126.vii ± 34.xviii | 0.41 |
| TSH (μIU/mL) | 3.94 ± 10.61 | ii.29 ± 2.xviii | 0.24 | 2.20 ± 1.49 | 1.96 ± i.xiii | <0.05 |
| T3 (ng/dL) | 98.72 ± xx.26 | 100.threescore ± 20.44 | 0.59 | 102.2 ± 18.60 | 103.two ± 16.44 | 0.40 |
| Free T4 (ng/dL) | 0.ninety ± 0.16 | 0.91 ± 0.13 | 0.71 | 0.94 ± 0.23 | 0.92 ± 0.13 | 0.21 |
Table iv. Associations between vitamin D status and biomarkers in men and women younger than 50 years.
Tabular array 4. Associations betwixt vitamin D status and biomarkers in men and women younger than fifty years.
| Men Younger than 50 Years | Women Younger than fifty Years | |||||
|---|---|---|---|---|---|---|
| Vitamin D < thirty (due north = 168) | Vitamin D ≥ 30 (due north = 82) | p Value | Vitamin D < xxx (n = 186) | Vitamin D ≥ xxx (north = 27) | p Value | |
| Age (years) | 41.58 ± 7.xi | 41.61 ± vi.08 | 0.98 | 41.97 ± 6.64 | 41.33 ± 6.27 | 0.64 |
| BW (kg) | 78.49 ± 13.39 | 76.52 ± 12.97 | 0.27 | 59.15 ± 9.99 | 55.77 ± iv.65 | 0.08 |
| BMI (kg/mii) | 25.88 ± 4.09 | 25.56 ± iii.97 | 0.57 | 22.81 ± 3.57 | 21.57 ± 2.04 | 0.08 |
| WBC (x3/μL) | 6.67 ± 1.lxxx | 6.41 ± i.72 | 0.28 | 5.88 ± i.lxxx | five.60 ± 1.61 | 0.20 |
| RBC (10six/μL) | 5.22 ± 0.50 | 5.19 ± 0.49 | 0.61 | 4.66 ± 0.43 | 4.46 ± 0.34 | <0.05 |
| Hb (m/dL) | xv.52 ± 1.31 | 15.65 ± 0.99 | 0.42 | 13.51 ± 1.46 | 13.72 ± 0.96 | 0.48 |
| Hct (%) | 45.88 ± 3.53 | 46.21 ± 2.59 | 0.44 | twoscore.35 ± 3.77 | 40.59 ± 2.96 | 0.75 |
| MCH (pg) | 29.87 ± two.77 | 30.34 ± two.53 | 0.19 | 29.17 ± 3.41 | 30.81 ± one.31 | <0.05 |
| MCV (fL) | 88.23 ± 6.95 | 89.55 ± 6.59 | 0.15 | 87.01 ± 8.32 | 91.eighteen ± 3.48 | <0.05 |
| MCHC (1000/dL) | 33.81 ± 0.91 | 33.86 ± 0.75 | 0.69 | 33.45 ± 1.08 | 33.80 ± 0.61 | 0.ten |
| Platelets (ten3/μL) | 236.five ± sixty.34 | 224.0 ± 48.86 | 0.16 | 268.2 ± 58.24 | 268.4 ± 85.49 | 0.99 |
| MPV (fL) | eight.44 ± 0.88 | 8.57 ± 0.88 | 0.26 | 8.46 ± 0.95 | 8.26 ± 0.90 | 0.30 |
| Fasting glucose (mg/dL) | 96.19 ± 15.71 | 93.73 ± 16.00 | 0.25 | 88.44 ± 19.96 | 88.15 ± eight.23 | 0.94 |
| HbA1c (%) | five.56 ± 0.sixty | 5.52 ± 0.75 | 0.65 | 5.33 ± 0.53 | 5.26 ± 0.28 | 0.48 |
| BUN (mg/dL) | 10.82 ± three.29 | 10.90 ± 3.xi | 0.85 | eight.29 ± two.39 | 7.37 ± ane.96 | 0.06 |
| Creatinine (mg/dL) | 0.95 ± 0.21 | 0.93 ± 0.12 | 0.42 | 0.65 ± 0.08 | 0.68 ± 0.08 | <0.05 |
| Uric acid (mg/dL) | 6.72 ± i.26 | six.55 ± 1.three | 0.32 | four.83 ± 0.99 | iv.94 ± 0.82 | 0.09 |
| AST (U/L) | 22.3 ± ten.71 | 24.37 ± 13.00 | 0.18 | 17.58 ± 6.58 | xvi.89 ± 2.99 | 0.59 |
| ALT (U/L) | 29.76 ± 21.73 | 30.20 ± 24.74 | 0.89 | sixteen.1 ± 12.03 | thirteen.11 ± 3.56 | 0.xx |
| r-GT (U/L) | thirty.93 ± 21.24 | 29.39 ± xix.97 | 0.58 | 16.45 ± ix.70 | 12.93 ± 4.68 | 0.06 |
| Alk-p (U/L) | 59.6± 15.68 | 55.88 ± 12.21 | 0.06 | 50.71 ± fifteen.01 | 42.07 ± 12.37 | <0.01 |
| Total bilirubin (mg/dL) | one.17 ± 0.55 | 1.19 ± 0.48 | 0.75 | 0.95 ± 0.40 | 0.96 ± 0.39 | 0.97 |
| Total protein (thousand/dL) | 7.21 ± 0.37 | 7.12 ± 0.36 | 0.06 | 7.eighteen ± 0.44 | 7.09 ± 0.42 | 0.32 |
| Albumin (g/dL) | four.58 ± 0.25 | 4.56 ± 0.24 | 0.50 | four.41 ± 0.25 | 4.42 ± 0.24 | 0.85 |
| Globulin (g/dL) | two.63 ± 0.35 | two.56 ± 0.34 | 0.14 | ii.77 ± 0.35 | 2.67 ± 0.36 | 0.17 |
| TG (mg/dL) | 151.i ± lxxx.28 | 134.ii ± 76.94 | 0.eleven | 106.7 ± 58.31 | 85.07 ± 26.82 | 0.06 |
| Total cholesterol (mg/dL) | 189.half-dozen ± 33.05 | 192.2 ± 39.57 | 0.59 | 188.7 ± 36.63 | 198.1 ± 37.69 | 0.21 |
| HDL-C (mg/dL) | 43.37 ± 8.89 | 49.2 ± thirteen.15 | <0.001 | 57.76 ± 13.45 | 67.74 ± 14.96 | <0.001 |
| LDL-C (mg/dL) | 129.0 ± 29.98 | 128.ane ± 36.74 | 0.85 | 117.seven ± 33.03 | 116.0 ± 32.25 | 0.80 |
| TSH (μIU/mL) | ii.00 ± ane.xiii | ane.92 ± ane.15 | 0.60 | ii.40 ±1.84 | i.87 ± 0.98 | 0.14 |
| T3 (ng/dL) | 107.4 ± 16.32 | 104.v ± fifteen.22 | 0.xviii | 99.41 ± xviii.75 | 98.78 ± 21.03 | 0.87 |
| Complimentary T4 (ng/dL) | 0.95 ± 0.13 | 0.94 ± 0.xiii | 0.51 | 0.93 ± 0.19 | 0.89 ± 0.xi | 0.29 |
Table 5. Associations between vitamin D status and biomarkers in men and women aged between 50 and 65 years.
Table five. Associations betwixt vitamin D status and biomarkers in men and women anile between 50 and 65 years.
| Men Aged between fifty and 65 Years | Women Aged betwixt l and 65 Years | |||||
|---|---|---|---|---|---|---|
| Vitamin D < 30 (n = 135) | Vitamin D ≥ xxx (due north = 100) | p Value | Vitamin D < 30 (n = 105) | Vitamin D ≥ 30 (n = 56) | p Value | |
| Age (years) | 56.55 ± 4.14 | 57.97 ± four.01 | <0.01 | 57.19 ± 3.98 | 58.05 ± 4.23 | 0.20 |
| BW (kg) | 74.95 ± 10.53 | 72.97 ± 9.31 | 0.13 | 57.36 ± ix.18 | 57.29 ± 7.63 | 0.95 |
| BMI (kg/chiliad2) | 25.96 ± 3.17 | 25.29 ± 2.8 | 0.09 | 23.02 ± 3.50 | 22.94 ± 2.89 | 0.87 |
| WBC (103/μL) | vi.36 ± 1.61 | vi.30 ± 1.69 | 0.74 | 5.55 ± 1.48 | 5.59 ± 1.84 | 0.88 |
| RBC (10six/μL) | five.11 ± 0.47 | five.08 ± 0.45 | 0.67 | 4.66 ± 0.32 | 4.67 ± 0.46 | 0.85 |
| Hb (g/dL) | 15.40 ± i.28 | 15.41 ± 1.32 | 0.92 | 13.90 ± ane.18 | 14.04 ± 0.92 | 0.45 |
| Hct (%) | 45.44 ± 3.48 | 45.55 ± 3.36 | 0.81 | 41.15 ± 2.96 | 41.75 ± ii.59 | 0.21 |
| MCH (pg) | xxx.24 ± two.57 | xxx.50 ± three.xiv | 0.50 | 29.91 ± 2.70 | 30.22 ± 2.23 | 0.47 |
| MCV (fL) | 89.25 ± 6.54 | 90.04 ± seven.54 | 0.39 | 88.54 ± 6.59 | 89.79 ± 5.63 | 0.23 |
| MCHC (grand/dL) | 33.85 ± 0.83 | 33.81 ± 1.06 | 0.70 | 33.74 ± 0.97 | 33.63 ± 0.78 | 0.47 |
| Platelets (103/μL) | 235.nine ± 72.80 | 223.iv ± 50.39 | 0.14 | 245.seven ± 53.23 | 240.viii ± 51.92 | 0.58 |
| MPV (fL) | 8.43 ± 0.88 | 8.47 ± 0.85 | 0.72 | viii.43 ± 0.85 | 8.44 ± 0.87 | 0.95 |
| Fasting glucose (mg/dL) | 105.ane ± 31.59 | 102.6 ± 25.36 | 0.52 | 96.04 ± 16.47 | 95.34 ± 17.58 | 0.80 |
| HbA1c (%) | v.95 ± ane.16 | 5.80 ± 0.81 | 0.28 | v.68 ± 0.52 | 5.64 ± 0.55 | 0.60 |
| BUN (mg/dL) | 12.00 ± 3.10 | 12.09 ± 3.17 | 0.83 | 10.62 ± 3.thirty | ix.91 ± 2.84 | 0.eighteen |
| Creatinine (mg/dL) | 0.94 ± 0.15 | 0.95 ± 0.15 | 0.71 | 0.67 ± 0.11 | 0.65 ± 0.10 | 0.46 |
| Uric acrid (mg/dL) | 6.44 ± i.27 | 6.39 ± one.24 | 0.76 | 5.21 ± 1.eleven | 5.07 ± 1.04 | 0.47 |
| AST (U/L) | 21.87 ± 7.62 | 21.80 ± half-dozen.82 | 0.94 | 21.04 ± 6.77 | 22.45 ± ix.40 | 0.28 |
| ALT (U/Fifty) | 27.91 ± sixteen.68 | 24.86 ± xiii.17 | 0.13 | 21.31 ± 11.67 | 23.08 ± 18.65 | 0.45 |
| r-GT (U/50) | 29.26 ± 17.68 | 33.53 ± 34.96 | 0.22 | 22.90 ± 18.12 | 20.23 ± 10.72 | 0.32 |
| Alk-p (U/L) | sixty.99 ± 24.11 | 56.98 ± 15.43 | 0.15 | 66.22 ± 17.00 | 61.50 ± fifteen.xc | 0.09 |
| Total bilirubin (mg/dL) | i.ten ± 0.46 | 1.11 ± 0.41 | 0.81 | 0.91 ± 0.36 | 0.95 ± 0.33 | 0.51 |
| Full poly peptide (g/dL) | 7.07 ± 0.38 | 7.04 ± 0.36 | 0.l | vii.18 ± 0.38 | vii.23 ± 0.41 | 0.51 |
| Albumin (thousand/dL) | 4.47 ± 0.25 | iv.47 ± 0.23 | 0.83 | 4.44 ± 0.24 | 4.42 ± 0.24 | 0.64 |
| Globulin (g/dL) | 2.60 ± 0.32 | ii.57 ± 0.31 | 0.54 | ii.75 ± 0.31 | 2.81 ± 0.34 | 0.25 |
| TG (mg/dL) | 161.6 ± 92.25 | 131.0 ± 65.82 | <0.01 | 117.1 ± 58.62 | 103.v ± twoscore.49 | 0.12 |
| Full cholesterol (mg/dL) | 185.ix ± 41.41 | 186.5 ± 37.74 | 0.91 | 211.five ± 41.89 | 215.0 ± 31.42 | 0.59 |
| HDL-C (mg/dL) | 44.eleven ± 9.56 | 46.38 ± 9.30 | 0.07 | sixty.56 ± 15.21 | 62.91 ± 17.49 | 0.38 |
| LDL-C (mg/dL) | 120.two ± 34.84 | 125.0 ± 35.79 | 0.31 | 133.viii ± 34.51 | 135.7 ± 27.04 | 0.72 |
| TSH (μIU/mL) | 2.10 ± ane.48 | 1.96 ± ane.thirteen | 0.44 | two.41 ±1.36 | two.00 ± 1.thirteen | 0.06 |
| T3 (ng/dL) | 103.3 ± 20.93 | 103.9 ± 15.73 | 0.lxxx | 97.89 ± 16.79 | 100.half dozen ± 16.45 | 0.33 |
| Gratuitous T4 (ng/dL) | 0.97 ± 0.40 | 0.94 ± 0.14 | 0.42 | 0.92 ± 0.14 | 0.92 ± 0.12 | 0.76 |
| Publisher's Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Artistic Commons Attribution (CC BY) license (https://creativecommons.org/licenses/past/4.0/).
friedmanorwil1946.blogspot.com
Source: https://www.mdpi.com/2075-4426/12/3/440/htm

 ,
,
0 Response to "Chao-Jen Huang Quizzes 440"
Post a Comment